Electron Diffraction
Introduction
⇒ Since waves behaved like particles, scientists wanted to know if particles could behave like waves
⇒ Diffraction is a clear property of waves, but if diffraction coud be obserbed using particles (e.g. electrons), then this would prove that particles also behave like waves
The Wavelengths of Particles
⇒ If particles behave like waves, they must have a wavelength
⇒ You can calculate the wavelength of a particle using the particle's momentum
⇒ The momentum of a photon is calculated using: Momentum (p) = mass (m) x speed of light (c)
⇒ P = mc can be substituted into the equation E = mc2 to give E = pc
- There is an 'mc' in E = mc2, which can be replaced by the 'p' from our momentum formula (E = p). We then need to add a 'c' to the formula to account for the fact the c is squared in E = mc2. Thus, we have E = pc
⇒ This formula can be rearranged to p (momentum) = E (energy) / c (speed of light)
⇒ Since E = hf, then p = hf⁄c = h⁄wavelength
⇒ It was proposed that this relationship would also be true for electrons (or any particle for that matter!)
⇒ Thus, he calculated the de Broglie wavelength to represent the wavelength of a movig particle: that is the Planck's constant divided by a particle's momentum
Diffraction Using Crystals
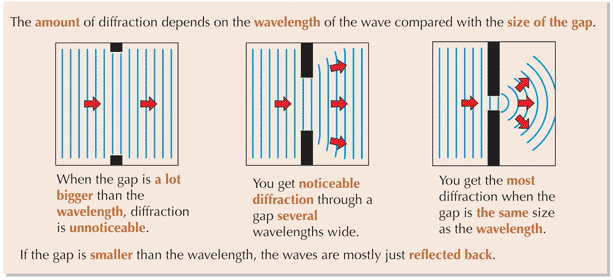
⇒ Diffraction is greatest when the wavelength of the wave is roughly equal to the gap it passes through
⇒ The wavelength of X-rays is about the same as the gap between ions within many crystals
⇒ Since the electron has a similar wavelength to X-rays, the same crystals should diffract both electrons and X-rays
⇒ In 1927, electron diffraction was seen for the first time
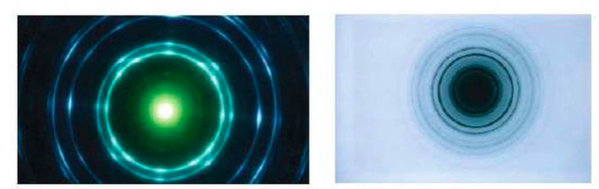
On the left you see x-ray diffraction and on the right you see electron diffraction
Calculating the de Broglie wavelength
⇒ The de Broglie wavelength is the wavelength of a moving particle
⇒ It is calculated as Planck's constant divided by the momentum of the particle:

⇒ The equation shows that faster moving particles have a shorter wavelength
Extra
⇒ Also see our notes on: