The Photoelectric Effect
What you should know
⇒ Electromagnetic waves are transverse and have a range of wavelengths/frequencies
⇒ Atoms are ionised when they gain or lose electrons
- Electromagnetic waves can ionise atoms when an electron absorbs a photon
- Electromagnetic waves with higher frequencies and lower wavelengths are more ionising
⇒ Electrons are negatively charged and have a mass about 2000 times larger than protons/neutrons
⇒ Energy is conserved - it cannot be created or destroyed
⇒ The energy of the photon is given by the formula: E = hf
Introduction
⇒ The photoelectric effect was first observed in the 1880s
⇒ At that time, scientists thought light was a wave, and this explaine many properties of light e.g. reflection, refraction, and defraction.
⇒ The photoelectric effect can be demonstrated using a gold leaf electroscope
The photoelectric effect is where electrons are emitted from certain materials when light (i.e. electromagnetic radiation) of a certain minimum wavelength arrives at the material
Gold Leaf Electroscope Experiment
⇒ In this experiment, you shine ultraviolet light (i.e. photons) on a zinc metal plate and can observe that electrons are emitted from the zinc (i.e. the photoelectric effect)
- Remember, photons are electromagnetic waves and have specific wavelengths, and therefore specific energies
- For the photoelectric effect to happen, the photons must have eough energy to break the electrons free from the atoms in the zinc metal plate
- Ultraviolet light has got the best wavelength (and therefore energy) to break the electron free from the atoms and give the electron enough kinetic energy to move away
⇒ Before conducting the experiment, you must negatively charge the gold leaf electroscope (i.e. this means that there are more negative electrons on the electroscope than positive protons)
- Obviously, because the leaves in the electroscope are both negatively charged, the leaves begin spread apart
⇒ Then, as you turn on the ultraviolet lamp, the photoelectric effect removes electrons from the zinc metal plate, which is in contact with the electroscope, thereby removing electrons from the electroscope
⇒ After a small amount of time, all of the extra negative electrons will be removed, and since the electroscope will no longer be negatively chaged (i.e. the number of electrons and protons are balancing out), the leaves come back together again
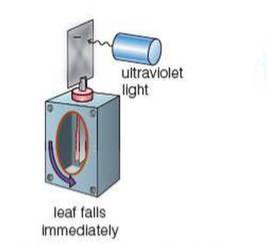
However
⇒ If the gold leaf electroscope begins with a positive charge, the electroscope will remain unchanged
⇒ Obviously, when it begins positively charge (like with the negatively charged gold leaf electroscope) the two gold leaves will begin spread apart (as they both have the same charge)
⇒ The fact that the ultraviolet light does not have any affect on the gold leaves shows that all the ultraviolet light did before was knock electrons out of the zinc metal plate
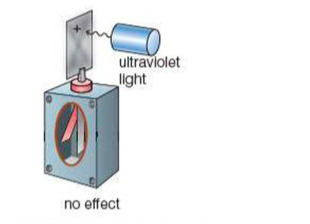
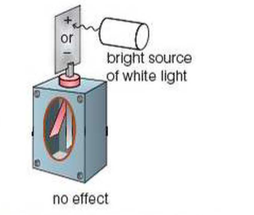
Other Light
⇒ If you use a white light or an intense red laser, for example, the electroscope will not be discharged
⇒ Scientists expected these other sources of light to emit electrons by thermionic emission (i.e. they thought these light sources would heat the zinc, providing the electrons in the atoms with enough energy to escape the atom's attractive forces and be emitted)
⇒ However, this was not the case
An Explanation With Quantum Theory
⇒ In 1900, Max Planck offered a solution to understanding the Photoelectric Effect
⇒ He said that light (and other electromagnetic radiation) is emitted in quanta (i.e. small packets of electromagnetic radiation, we now know as photons)
⇒ He said that each individual quantum (i.e. packet) of light contains energy that is proportional to its frequency (E = hf)
⇒ This is how the solution works:
- For an electron to be removed, it must absorb a certain minimum amount of energy
- Planck suggested that a photon from the light interacts with an electron, which absorbs the energy that was contained within the photon
- The ultraviolet photons have enough energy to knock the electrons out of the metal i.e. electrons are removed and the electroscope is discharged
- However, photons of visible light (for example) have a lower frequency (and, therefore, a lower energy), so when the electron absorbs these photons there is not enough energy for the electron to escape from the metal (and, therefore, the electrscope is NOT discharged)
Further Photoelectric Experiments
⇒ Other experiments can be used to demonstrate the photoelectric effect too, and a number of experimental observations have been made:
- No photoelectrons are emitted if the frequency of light is below a certain value. This is called the threshold frequency
- The threshold frequency varies for different materials. Visible light removes photoelectrons from alkali metals, and calcium and barium. Other metals require ultraviolet radiation, which has a higher frequency than visible light.
- More photoelectrons are emitted as the intensity of light increases, but only if the frequency of the light used is above the threshold value.
- Photoelectrons, emitted from a particular metal, have a range of energies. Their maximum kinetic energy depends on the frequency of the incident light so long as the frequency of this light is above the threshold frequency.
A model to explain the photoelectric effect
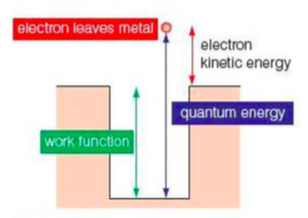
⇒ To explain threshold energy (i.e. the energy required to cause the photoelectric effect), we can imagine the the electrons in the metal being trapped in a potential well
- The depth of the well represents the least amount of energy needed for the electron to escape from the metal (that is the threshold energy)
- The electron gains this energy by absorbing photons. If the energy it absorbs is above the threshold, it will escape from the potential well and will be released as a photoelectron
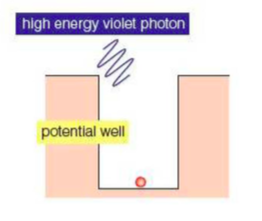
⇒ The energy needed to escape from the potential well is different for different metals
- E.g. in alkali metals the potential well is small, so the threshold energy is small and photoelectrons are released using visible light
- Electrons at the surface require less energy to escape compared to electrons deeper within the metal
⇒ If a photon does not reach the threshold frequency then it will not have enough energy to release a photoelectron and the photoelectric effect ill not be observed
- Electrons cannot be released by absorbing two photons, in the same way you cannot escape out of a hole by taking two small jumps
- Each photon must carry at least the threshold energy in order to release an electron
⇒ The photon's energy does work to release the photoelectron and give it kinetic energy
Einstein's Photoelectric Equation
⇒ As energy is conserved, the energy within the photon is equal to the threshold energy + the kinetic energy of the photoelectron
- This is reflected in the equation below
⇒ The work function of a material is the least energy needed to release a photoelectron from a mateiral (this equals the threshold energy). The work function has the symbol Φ
⇒ The energy within a photon is equal to hf and this energy is transferred to the electron to release it from a material (the work function) and give the released photoelectron some kinetic energy:
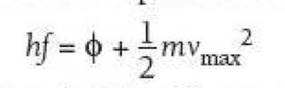
- h = Planck's constant
- f = the frequency of the radiation
- Φ = the work function of the material in joules
- 1/2 mvmax2 = the maximum kinetic energy of the photoelectrons
⇒ This is Einstein's photoelectric equation
Using the work function
⇒ When a material's work function is less than 3.1 eV, visible light can release electrons
⇒ When a material's work function is less than 1.7 eV, infrared light can release electrons
⇒ Semiconductors are mateirals that have been treated to have a low work function, such that visible light and infrared light enable the photoelectric effect e.g. in solar cells
Stopping Potential
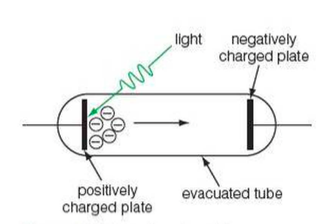
⇒ This shows a photoelectric cell
⇒ When light hits the metal surface on the left, some photoelectrons are emitted
⇒ These emitted photoelectrons can be detected by a sensitive ammeter when they reach the electrode on the right
⇒ By giving the electrode on the right a negative charge, instead of a positive charge, the photoelectrons can be turned back so they don't reach the electrode at all
⇒ Because the electrons don't reach the negatively charged electrode, the current will be zero as there is no flow of charge - a stopping potential has been applied to the electrons
⇒ This stopping potential gives a measure of the electrons' maximum kinetic energy
- If the electrons have alot of kinetic energy, they can overcome the repulsive force
- But, if we turn up the reverse voltage until the electrons with the most kinetic energy are just repelled, that voltage is called the stopping voltage or stopping potential
- So we can say at this point that the work done by the electric field to stop the electron (in eV) is equal to the photoelectron's kinetic energy
⇒ Thus, the electron's kinetic energy may be calculated using this equation:
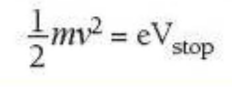
Extra
⇒ Also see our notes on: