Moving and Storing Heat
Introduction
⇒ Heating is all about transferring energy
Heat Measures Energy
⇒ By heating something, the particles it contains gain energy
⇒ As a result, in solids and liquids, the particles start moving around faster. And, in a solid, the particles vibrate more rapidly.
⇒ Energy is measured in joules
Energy Transfer
⇒ Energys tends to move from a hot object to a colder one. For example, radiators heat up the cooler air in a room.
⇒ The larger the difference of temperatures between the object (e.g. the radiator and the room), the faster heat will be transferred
- In other words, where temperatures differ between objects, energy will flow
Specific Heat Capacity
⇒ Some objects require a greater amount of heat energy to increase its temperature compared to other objects
- For example, 4200 joules of energy is needed to heat 1 kilogram of water by 1 degrees celsius, but you only need 139 joules of energy to increase 1 kilogram of mercury by the same amount
⇒ Materials that take a lot of heat energy to get hot, also release a lot of heat energy when cooling - in other words, they can 'store' a lot of heat
⇒ The specific heat capacity is a measure of how much energy something can store
- More precisely, the specific heat capacity is a measure of the amount of energy is needed to heat 1 kilogram of something by 1 degree celsius - thus, water has a specific heat of capacity of 4200 J/kg°C
⇒ Water has a high specific heat capacity, which means it stores a lot of energy making it perfect for central heating in your house
⇒ This is an important equation to know:
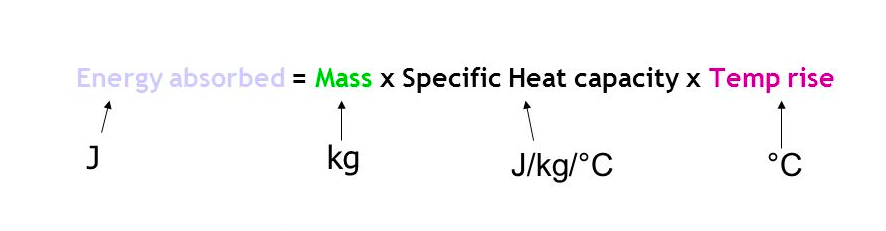
Example
⇒ 1. How much energy is needed to heat 2 kilograms of water from 10°C to 100°C?
- Energy needed = 2 x 4200 x 90 = 756,000J
⇒ 2. An empty 200g aluminium kettle coolds down from 115°C to 10°C, losing 19,068J of heat energy. What is the specific heat capacity of aluminium?
- SHC = Energy⁄Mass x Temp Change = 19,068⁄0.2 x 105 = 908 J/kg°C