Excitation and Ionisation
Introduction
⇒ If all the electrons are in the lowest energy level, we say the atom is in its ground state
⇒ If the electrons in an atom absorb energy and move to higher energy states, we say the atom is excited
- Excitation only occurs if the electron absorbs exactly the right amount of energy to move to higher energy levels
⇒ Excitation will occur in the following circumstances:
- An electron absorbs a photon with exactly the right amount of energy to move it between two levels (so a photon will not be absorbed if the energy it provides to the electron is not enough for it to move between two levels)
- An electron absorbs exactly the right amount of energy to move between two levels after colliding with a free electron (that contains the same energy or greater energy than that required for the electron to change energy levels).
- As energy is conserved, the energy gained by the electron in the atom equals the energy lost by the free electron
- The free electron's kinetic energy after the collision is the same as before the collision minus the energy transferred to the excited electron in the atom
Ionisation
⇒ If an electron in an atom absorbs enough energy to leave the atom completely, the atom is said to be ionised
⇒ By gaining or losing an electron, it becomes a charged particle (i.e. an ion)
⇒ The ionisation energy is the minimum energy needed to remove the electron from the atom completely
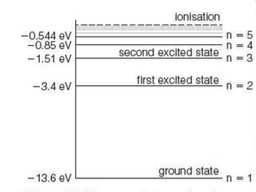
⇒ This shows a number of possible excited states for a hydrogen atom
Line Spectra and Continuous Spectra
⇒ When light passes through a small gap it is diffracted (i.e. it spreads out)
⇒ A diffraction grating is a piece of transparent material ruled with very closely spaced lines, used to see the diffraction of light
- As light passes through, it is split into a spectrum of the colours it contains
- The pattern of the coloured light produced carries information about the light source

⇒ Sunlight and the light produced by a normal incandescent bulb gives a continuous spread of colours that merge into one another (i.e. they have a continuous spectrum)
- The reason why icandescent bulbs have a continuous spectrum is because they work by heating a solid filament
- As energy levels in a solid overlap, all energy changes for the electrons are allowed - so the electrons can emit photons with any energy
⇒ However, when light is produced using a fluorescent light source a line spectrum is produced i.e. a spectrum of discrete coloured lines of light
- All fluorescent lights have a similar line spectrum because the electrons in the mercury vapour inside the bulbs have the same excited states
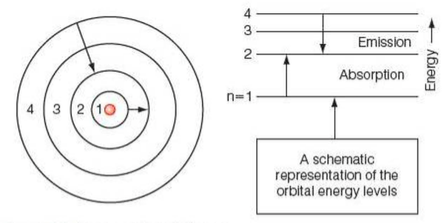
Emission Spectra
⇒ An emission spectrum is a bright spectrum seen when electrons in atoms fall from higher energy levels to lower energy levels, releasing photons
- In gas (such as the mercury vapour inside the fluorescent bulb), electrons can only occupy a small number of discrete energy levels
- When the gas is heated, the electrons absorb energy and move to higher energy levels
- However, the electron doesn't stay in the higher energy for long and soon returns to the lower energy level
- When the electron moves back to the lower energy level, a photon is emitted containing an amount of energy equal to the difference in energy between the two energy levels that the electron has been occupying
⇒ The photons emitted through this process have specific energies because only a specific set of energy levels exist for the electron to move between (this also means that the photons have specific wavelengths and colours)
⇒ It is these specific colours that we see as lines when the light is diffracted through a diffraction grating
Absorption Spectra
⇒ An absorption spectrum can be seen when a light (i.e. photons) shines through a gas, and electrons within the gas absorb photons that correspond exactly to the energy transition required for the electron to move up an energy level
- All other photons won't be absorbed and will simply go straight through
⇒ The absorption spectrum, therefore, is the spectrum of dark lines produced when an electron absorbs a photon
- Although soon after the absorption the photon will be emitted from the electron again (and the electron will move back down an energy level), the spectrum will still have the black lines on it
Fraunhofer Lines
⇒ Fraunhofer realised that the sun's continuous spectrum was overlaid with 570 dark absorption lines (i.e. Fraunhofer lines)
⇒ Processes within the sun create a continuous spectrum of radiation, and this passes through cooler gasses in the outer layers of the sun
⇒ Electrons within the cooler gasses absorb the photons at specific frequencies (so the electron moves up an energy level) and then re-emit them at different frequencies (and so the electron goes back down an energy level, to its ground state)
The Energy of Spectral Lines
⇒ When an electron moves to a lower energy level, it emits a photon
⇒ The energy lost by the electron in this process equals the energy of the photon: E2 - E1 = hf
- E1 and E2 are the energies of each energy level (in Joules), h is Planck's constant (in Js) and f is the frequency of the radiation (in Hz)
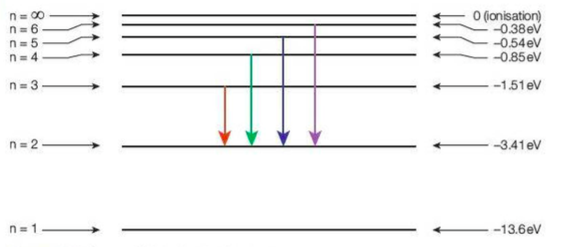
⇒ This shows the energy levels in a hydrogen atom:
- The ground state is labelled n = 1, and the first excited energy level is n = 2 and so on
- The ionisation energy is 12.6 eV (moving the electron from its ground state to n = ∞)
- The arrows indicate the electron moves to a lower energy level, emitting photons. An emission spectrum would be seen
- The energy is always negative as we define the zero point of energy is when the electron is at infinity. This energy gets lower as the electron gets closer to the nucleus
- The energy of the green line is the difference in energy between n = 4 and n = 2, that is -0.85eV - (-3.41 eV), or 2.56 eV (4.1 x 10-19 J)
Extra
⇒ Also see our notes on: