The Fluorescent Tube
Overview
⇒ Fluorescent lighting is quite common, especially in schools and universities
⇒ Fluorescence in these lights occurs when electrons absorb photons of ultraviolet radiation and move to a higher energy level
⇒ When the excited electrons fall back to the lower energy level, energy is released as visible light
Fluorecent Tube
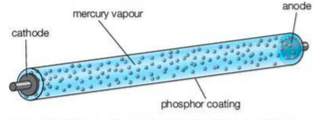
⇒ The fluorescent tube is a glass tube filled with mercury vapour and coated inside with fluorescent materials (i.e. phosphors)
⇒ When you turn the light on, the cathode is heated causing thermionic emission
- Thermionic emission happens when free electrons are emitted from a heated filament (i.e. the cathode here)
⇒ A potential difference of 500V across the tube causes these electrons to accelerate from cathode to anode through the mercury vapour
⇒ If the electrons collide with the mercury atoms, energy may be transferred causing ionisation or excitation (as long as these free electrons provide enough kinetic energy to do so)
⇒ As the mercury atoms become ionised (i.e. lose their electrons), a mixure of ions and free electrons is created within the gas (i.e. plasma)
⇒ When the electrons in the excited mercury atoms return to their ground state, they release photons of ultraviolet radiation
⇒ These photons strike the phosphers in the coating - the energy is re-emitted as visible light, and some energy is transferred as heat
Extra
⇒ Also see our notes on: