Introduction to Electrons and Energy Level
What you should already know
⇒ Energy is measured in joules
⇒ Charge (Q) is measured in coulombs (C)
- A single electron carries a a charge of 1.6 x 10-19 C
- Current (I) - measured in Amperes (A) - is the rate of flow of charge
- A current of 1A means that one full coulomb of charge flows past a point in a circuit every second
- Current is measured using an ammeter
- Potential difference (i.e. voltage) is measured in volts (V)
- Potential difference is the work done per unit charge
- When a force moves an object, energy is transferred and work is done (so work is measured in joules)
- Therefore, a potential difference of one volt transfers 1 joule per coulomb of charge: 1V = 1JC-1
⇒ In an atom, the central nucleus is positively charged and the surrounding electrons are negatively charged
⇒ A neutral atom has the same number of protons and electrons. It becomes ionised when it gains or loses electrons
- If the atom loses an electron, it gains charge. If the atoms gains an electron, it loses charge
⇒ There is a spectrum of electromagnetic radiation
- Visible light is one form of electromagnetic radiation
- Higher frequency radiation (such as gamma rays) have a shorter wavelength, compared to low frequency radiation (such as microwaves) which has a longer wavelength
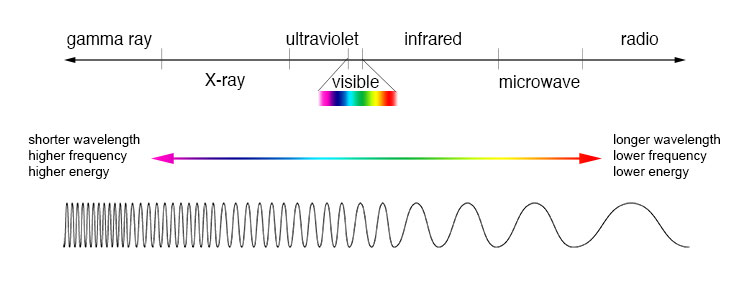
⇒ In order of decreasing wavelength (i.e from long to short) the electromagnetic spectrum is as follows: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, gamma rays
Overview
⇒ Electromagnetic radiation carries information across the universe at the speed of light
⇒ Every chemical element has a unique 'signature' (i.e. emission spectrum) which can be revealed by analysing the light it gives off by spreading the light out into a spectrum
- For example, the spectrum from a star can be analysed to reveal what elements are contained within the star itself
⇒ Light from distant galaxies takes billions of Earth to reach us, by which time the spectrum from the galaxies has red shifted (i.e. the wavelength of the light has been lenghtened), meaning we can tell the speed and distance of each galaxy from Earth (and, therefore, the age of the universe)
⇒ Line spectra can be used for many different things e.g. identifying samples of illegal drugs
Extra
⇒ Also see our notes on: