Photons
Introduction
⇒ Electromagnetic radiation often behaves as a wave
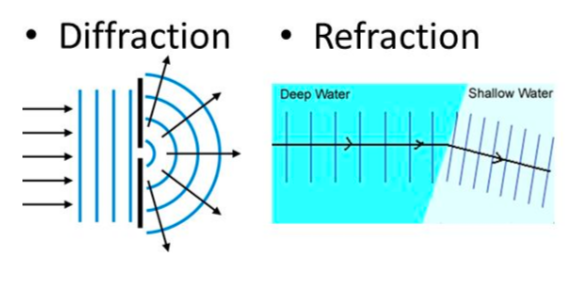
- E.g. waves change wavelength and speed at boundaries between different materials, such as when light goes into water (i.e. refraction), and waves can spread through gaps and around corners (i.e. defraction)
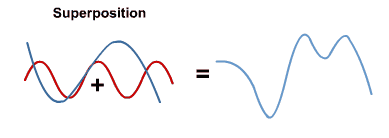
- When electromagnetic waves in the same region overlap, their amplitudes add (superposition)
⇒ However, other behaviours can only be explained by thinking of electromagnetic radiation as a stream of packets (i.e. quanta) of energy, called photons
- A photon has no mass or charge
- We describe a photon by its energy, wavelength or frequency e.g. xray photons have a wavelength of about 1nm and frequency of 3 x 1017 Hz. Energy of photons is seen below.
Energy of Photons
⇒ The energy of a proton is proportional to its frequency e.g. the energy of an xray photon is higher than a photon of visible light because the xray photons have a higher frequency
⇒ To calculate the energy of a photon we use the following formula: E = hf
- E = the energy (measured in joules)
- h = Planck's constant (6.63 x 10-34 Js)
- f = the frequency of the photon (measured in hertz)
⇒ Since frequency x wavelength = wave speed, we can also write the equation as follows: E = hc⁄wavelength
Intensity
⇒ The intensity of electromagnetic radiation is the energy transferred per unit time per unit area
⇒ Electromagnetic intensity depends on the energy carried by the photons, the number of photons transferred each second, and the area on which they are incident
⇒ So, the intensity of electromagnetic radiation will increase in the following situations:
- Where the light source is more powerful, so more photons are transferred per second e.g. the intensity of a 100W bulb is greater than that of a 10W bulb
- Where each photon carries more energy e.g. an ultraviolet light photon carries more energy than a visible light photon
- Where the light is incident on a smaller area - so as you move closer to the light source the intensity increases. The intensity has an inverse-square relationship to distance
The Electron Volt
⇒ Electrons within an atom can absorb photons and gain energy
- An electron that gains energy may move to a higher energy level away from the nucleus or leave the atom completely
⇒ Energy carried by photons and gained by electrons is extremely small, so a different unit of energy is used - the electron volt (eV)
- An electron volt is a unit of energy equal to 1.6 x 10-19 J. It is the energy needed to move an electron through a potential difference of 1V
- So work is done moving electrons in an electric field
⇒ The work done in electron volts (i.e. the energy transferred) is calculated using the following formula: W = VQ
- W - the energy transferred in electron volts
- V - the potential difference in volts
- Q - the electron charge (1.6 x 10-19 C)
⇒ To convert electron volts to joules, multiply the energy in electron volts by 1.6 x 10-19 J/eV
⇒ To convert joules to electron volts divide the energy·in joules by 1.6 x 10-19 J/eV
Absorbing and Emitting Photons
⇒ An electron gains and loses energy as it moves within the atom
⇒ The electron contains both kinetic energy and electrostatic potential energy
⇒ The positive charge of the proton means that the negatively charged electron is attracted to the nucleus, so work must be done to move the electron away from the nucleus (hence why the electron has less energy as it gets closer to the nucleus)
⇒ The electron will move further from the nucleus (i.e. move to a higher energy level) if it gains the right amount of energy by absorbing a photon
⇒ On the other, if the electron loses energy by emitting a photon it will move closer to the nucleus (i.e. move to a lower energy level)
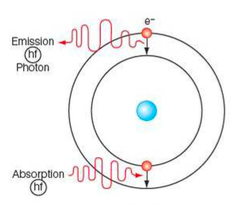
Quantised Energy Levels
⇒ An electron can only absorb a specific amount of energy (i.e. the possible or allowed energies for electrons are not continuous)
- In other words, these energy levels are quantised energy levels because they have fixed energy values
⇒ For example, when you use stairs you can only gain or lose gravitational potential energy in fixed amounts
- However, although stairs tend to have the same spacing between each level, the arrangement of energy levels in atoms is far more complex
Extra
⇒ Also see our notes on: