The Building Blocks of the Universe
Models in Physics
⇒ We use models to explain how things around us work
⇒ Some models use everyday objects around us, to help explain concepts more simply. For example, the Rutherford-Bohr model envisions an atom with a small ball for a nucleus and even smaller balls (electrons) wizzing around the outside.
⇒ Other models are based largely on maths. For example, the kinetic theory of gas shows gas molecules behaving like bouncing balls and colliding with each other in all directions
⇒ A model is good when it can adapt and change to new discoveries
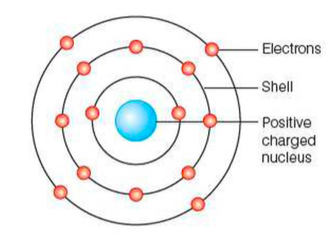
The Rutherford-Bohr model
⇒ JJ Thompson was the first person to create a model for the atom (the plum pudding model) but this was soon replaced by the Rutherford-Bohr model
⇒ With the Rutherford-Bohr model there is a positively-charged ball in the centre consisting of protons and neutrons, and it is surrounded by smaller, negatively-charged, electrons
Rutherford Scattering
⇒ To work out the size of the nucleus, Rutherford directed beams of alpha particles (which are the nuclei of helium atoms and hence positively charged) at thin gold foil
- The gold foil had an atomic radius (i.e. the distance from the centre of the nucleus to the boundary shell of electrons) of 134pm (picometres) - 1pm is 1 x 10-12m, so it's extremely small
⇒ They discovered, by doing this, that the radius of the nucleus of gold was approximately 27fm (fentometres or fermi) - 1fm is 1 x 10-15m
⇒ However, today scientists believe that the radius of the nucleus of gold is closer to 5fm, making it about 25,0000 times smaller than the radius of the gold atom itself
Charges, Masses, and Specific Charges
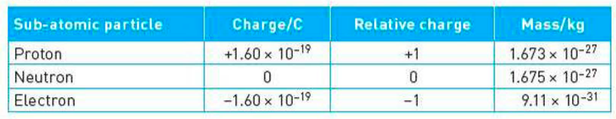
⇒ The specific charge of a particle is simply the ratio of its total charge to its total mass
⇒ It is defined as charge per unit mass (Ckg-1)
⇒ The formula for specific charge is as follows:
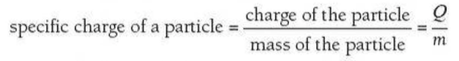
⇒ For example, if you wanted to calculate the specific chage of a proton you would take the charge and mass of the proton from the table above and put it into our equation:
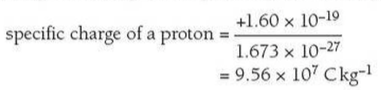
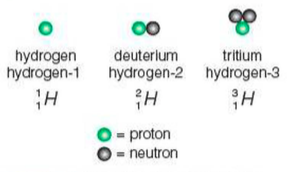
Describing Nuclei and Isotopes
⇒ The proton number tells you how many protons there are in a given nucleus
⇒ The nucleon number tells you the total number of protons and neutrons in a nucleus
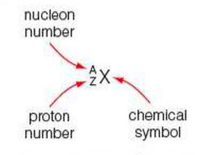
⇒ NOTE: this is different to chemistry and the periodic table as they use A and Z to describe atoms rather than the nucleus.
⇒ This AZ notation is good, because it allows us to describe isotopes too where the proton number (Z) will stay the same (and, therefore, so will the chemical symbol), but the nucleon number (A) will change as the number of neutrons differs in isotopes
⇒ For example, see how three different isotopes of hydrogen are written: