Introduction to Particles and Nuclides
What You Should Know
⇒ Everything around you is made of atoms, which include a nucleus (made of protons and neutrons) and electrons which orbit the nucleus
- The proton/atomic number tells you how many protons are in an atom's nucleus
- The nucleon/mass number tells you the total number of protons and neutrons, together, in the nucleus of the atom
⇒ Protons have a positive charge, electrons have a negative charge, and neutrons have a neutral electric charge
⇒ Atoms are always electrically neutral, so an atom must have the same number of protons and electrons
- However, an ion is formed where the atom gains or loses an electron.
- If the atom gains an electron that would make the atom a negatively charged ion, but if it loses an electron it would become a positively charged ion
⇒ The nucleus is tiny compared to the atom itelf (about 10,000 times smaller in diameter than the atom's diameter). However, it is also the heaviest part of the atom
- While protons and neutrons are similar in size (a relative mass of 1), electrons are 1800 less massive
⇒ An element is a substance that is made entirely from one type of atom e.g. the element hydrogen is made from atoms containing just one proton and one neutron - all such atoms, therefore, have the same proton number
- An isotope, on the other hand, contains atoms with the same number of protons but a different number of neutrons
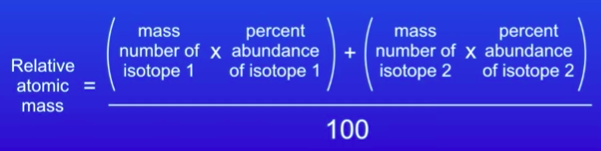
⇒ The carbon-12 atom, is the standard atom against which the masses of other atoms are compared
- The relative atomic mass is the ratio of the average mass of one atom of an element to one twelfth of the mass of an atom of carbon-12.
Extra
⇒ Also see our notes on: