Stable and Unstable Nuclei
Why is the nucleus stable?
⇒ You would think a group of positively charged protons (in the nucleus) would repel each other, but they don't
⇒ This means there needs to be some attractive and short range force that is stronger than the repulsive force between the protons
⇒ This strong force must also apply equally between protons and neutrons, otherwise it would be very easy to remove neutrons from the nucleus
⇒ Finally, at extremely short distances there needs to be a repulsive force otherwise the protons and neutrons (i.e. nucleons) would collapse and implode in on themselves!
The Strong Nuclear Force
⇒ The strong nuclear force is one of the four fundamental forces of nature. It is a very short-range force and acts between nucleons (protons and neutrons) holding nuclei together and keeping them stable.
⇒ This strong nuclear force is attractive at distances between 0.4 fm (femtometres) and 3 fm (3 x 10-15
⇒ Below 0.4 fm the force is repulsive
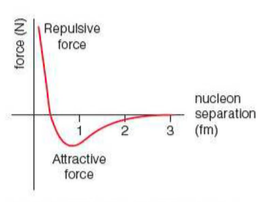
⇒ As you can see from the graph, when the distance is 0.4fm the force is neither attractive or repulsive - where the nucleus is stable, this is the separation of each nucleon
- At this distance, the resultant force on each nucleon is zero
⇒ Also note that when the distance is above 3fm the strong nuclear force drops to 0 too
Alpha and Beta Radioactive Decay
⇒ Not all nuclei are stable
⇒ Radioactive decay happens in unstable atomic nuclei - that is ones that don't have enough binding energy to hold the nucleus together due to an excess of either protons or neutrons
- This is why the vast majority of isotopes are unstable
⇒ The three most common decay mechanisms are alpha, beta, and gamma decay
Alpha Radiation
⇒ With alpha radiation, alpha particles are emitted
⇒ An alpha particle consists of two protons and two neutrons (this is identical to the nucleus of a helium atom)
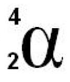
⇒ The symbol for an alpha particle can be seen here - it has a proton number of 2 and a mass number of 4
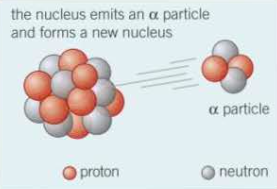
⇒ In this diagram, there is an unstable nucleus on the left, from an element, emitting an alpha particle
⇒ When the alpha particle is emitted, the nucleon number (total number of protons and neutrons) decreases by 4 and the atomic number (number of protons) decreases by 2 in the unstable nucleus
⇒ This change in the number of protons and neutrons will cause the decaying element to transform into a new element
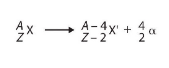
Beta Radiation
⇒ In shot, beta radiation involves the emission of an electron
⇒ We represent this emitted electron (i.e. our beta particle) using the following symbol:
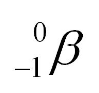
⇒ When an unstable nucleus of an element emits a beta particle the following happens:
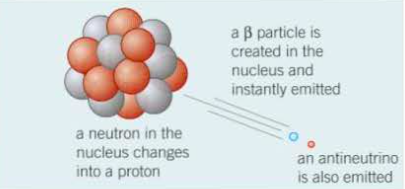
⇒ Essentially, a beta particle (i.e. an electron) is emitted instantly when a neutron in the unstable nucleus changes into a proton
⇒ In addition to this, an antiparticle with no charge, called an antineutrino  is emitted
is emitted
- See our notes on antiparticles and neutrinos for more detail
⇒ The transformation of a neutron into a proton increases the atomic number by 1, but the nucleon number stays the same, causing the decaying element to transform into a new element
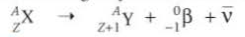
Gamma Radiation
⇒ Gamma radiation is electromagnetic radiation emitted by an unstable nucleus
⇒ It has no mass and no charge
⇒ It is emitted from the nucleus where that nucleus has too much energy, following the emission of alpha or beta particles
Beta Particle Spectrum Graph
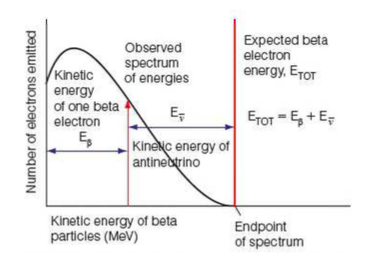
⇒ During beta radiation a certain amount of energy is emitted. The total energy emitted during beta radiation (ETOT) is made up of the kinetic energy from the beta particle plus the kinetic energy from the antineutrino
⇒ This graph shows that there is not a single unique kinetic energy for the β− particle
⇒ The observation of a continuous range of β− particle kinetic energies in a given β– decay process is because of the involvement of antineutrinos